
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder and the most common form of dementia, accounting for 50 to 70 percent of worldwide dementia cases. Decades of researches have pathologically characterized AD by two proteinaceous aggregates: amyloid plaques composed of amyloid-β (Aβ) peptides and neurofibrillary tangles consisting of the hyperphosphorylated microtubule-associated protein tau. Now researchers from Chinese Academy of Sciences unraveled a novel mechanism regulating the amyloidogenic processing of Aβ precursor protein (APP) and provided a potential therapeutic strategy against AD.
Dr. PEI Gang, Dr. ZHAO Jian and their colleagues from the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences found that β-arrestin1, a multifunctional protein that mediates G protein-coupled receptor (GPCR) desensitization, internalization and initiates G-protein independent signaling of GPCRs, is a critical modulator of Aβ pathogenesis and could be a potential target against AD. By a close investigation with the brain samples of sporadic AD patients provided by Netherland Brain Bank and the brains of transgenic AD model mice, they found that the expression of β-arrestin1 was upregulated and correlated well with neuropathological severity and senile Aβ plaques, indicating a physical relevance of β-arrestin1 with AD pathogenesis. Knockout of β-arrestin1 expression in transgenic AD model mice diminished Aβ pathology and behavioral deficits in these AD mice.
Their mechanistic study reveals that β-Arrestin1 enhances γ-proteolysis of APP via its direct interaction with APH-1, thereby modulating Aβ production in vitro and in vivo. By binding to the γ-secretase subunit APH-1, β-Arrestin1 facilitated the NCT/APH1 precomplex and mature γ-secretase complex formation. Thus, deficiency of β-arrestin1 or inhibition of binding of β-arrestin1 with APH-1 by small peptides reduced Aβ production. Moreover, elimination or partial reduction of β-arrestin1 regulatory function on γ-proteolysis of APP ameliorates Aβ pathological features without completely inhibiting γ-secretase activity or affecting the proteolysis activity of γ-secretase to other substrates including Notch, which indicates a preservation of the normal physiological functions of γ-secretase.
This study not only identifies a regulatory mechanism underlying both γ-secretase assembly and AD pathogenesis, but indicates specific reduction of Aβ pathology can be achieved by regulation of the γ-secretase assembly, suggesting that targeting β-arrestin1 and/or its interaction with APH-1 may implicate an appealing avenue for new preventive or therapeutic strategies against AD.
This study entitled "β-Arrestin1 regulates γ-secretase complex assembly and modulates amyloid-β pathology” has been published online on Cell Research on Dec. 4th.
CONTACT:
PEI Gang
Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences,
Shanghai, China
Phone: 86-21-54921371
E-mail: gpei@sibs.ac.cn
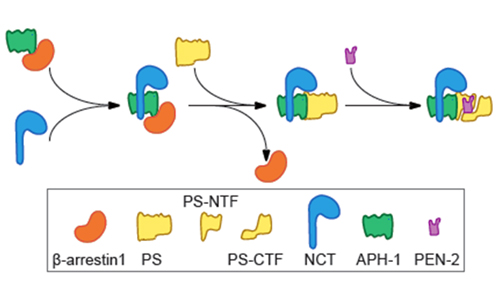
Mediation of γ-secretase complex assembly by β-Arrestin1 (Image by Dr. ZHAO Jian)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

